Difference between collision theory and transition state theory Collision theory concentration difference between transition state particles high gas volume collisions colliding then results vs terms The collision theory
Collision Theory | General Chemistry
What is the collision theory of chemical reactions? Collision collisions molecular byjus (pdf) transition state theory for collision complexes: product
Collision theory reaction chemical rates effect chemistry reactions model rate temperature orientation energy kinetics atoms collisions colliding molecules effective molecular
Theory transition state collision chemistry apAp chemistry collision theory/transition state theory Collision theoryCollision theory reaction rate kinetics temperature energy kinetic effect concentration changes time level compared.
Collision chemicalCollision theory collisions molecular chemistry energy model examples activation Collision theoryCollision theory.

Theory collision
Collision theory chemistry openstax levelTransition theory collision difference Collision molecules orientation chemistry atoms chemical collisions reactions oxygen activation collide colliding monoxide kinetics occur molecule atom chem chapter mightCollision theory.
Collision theory chemistry molecular examples collisionsCollision theory Collision theory not conditions these if ppt powerpoint presentation collide forming merely bounce without another off particles met both willCollision activated summary.

Reaction rates collision theory chapter diagram effective rate ppt powerpoint presentation collisions
Theories rate ashika collisionDifference between collision theory and transition state theory Difference between collision theory and transition state theoryTheories of reaction rate.
Collision theory & transition state theory(l-11) chemical kineticsTheory transition collision state reprinted distributions chemical physics complexes translational letters energy Collision theoryCollision molecules collisions chemistry theory reaction carbon oxygen orientation between two diagram colliding atom figure molecule monoxide occur take atoms.

Collision theory
Collision concentration6.1 collision theory (sl) Collision theoryCollision theory reaction chemical reactions energy rates kinetics explain activation rate collisions orientation particles molecules does occur equilibrium colliding chemistry.
Theory collision reaction rates ppt powerpoint presentationDifference between activated complex theory and collision theory Collision theory.


Kinetics - Collision Theory (A-Level) | ChemistryStudent

(PDF) Transition state theory for collision complexes: Product

Collision Theory | A-Level Chemistry Revision Notes

PPT - Collision Theory PowerPoint Presentation, free download - ID:918017

What is the collision theory of chemical reactions? | Socratic
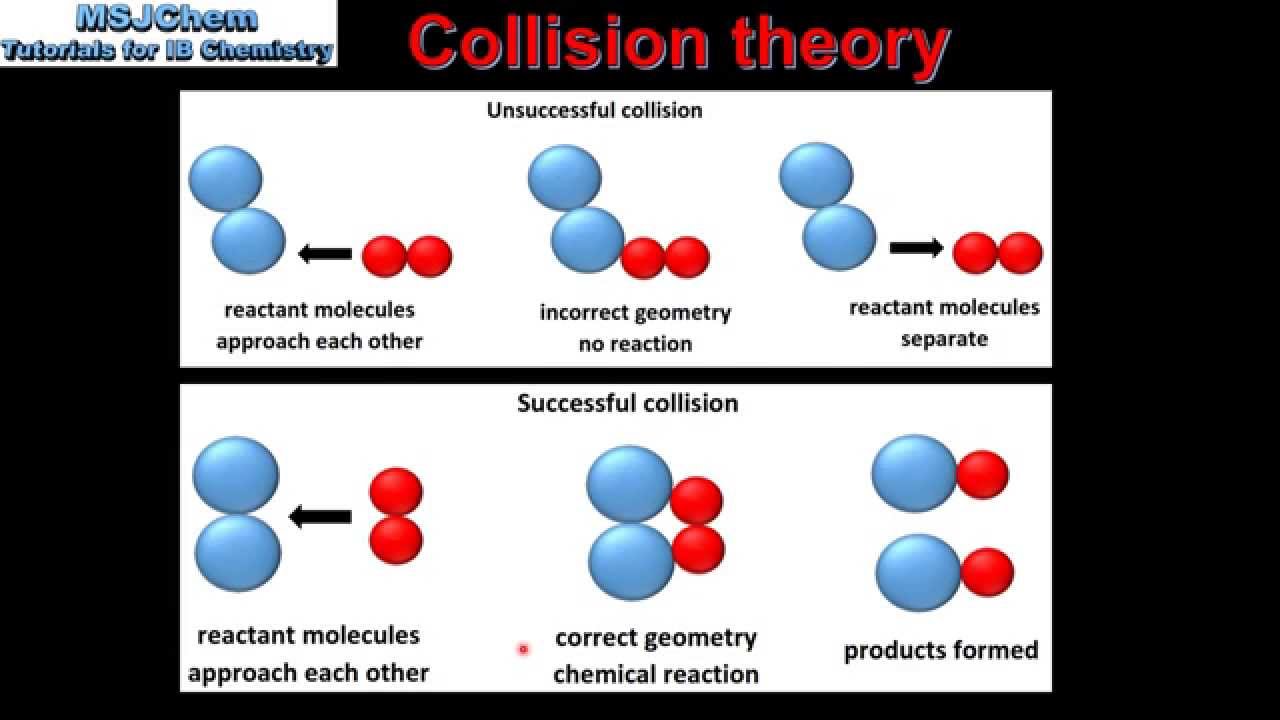
6.1 Collision theory (SL) - YouTube

collision theory & transition state theory(L-11) chemical kinetics

Theories of Reaction Rate - Ashika G